ICH Q14, on analytical procedure development, is set to revolutionize the pharmaceutical industry. This guideline provides a structured framework for developing and validating analytical methods, ensuring accuracy, consistency, and regulatory compliance. By streamlining processes and enhancing data quality, ICH Q14 will drive innovation and efficiency in drug development and manufacturing.
What is the ICH Q14 guideline?
ICH Q14 guideline describes the science and risk-based approaches for developing analytical procedures for testing the quality of drug substances and products. Testing procedures can apply to all release and stability testing of commercial drug substances and final drug products and testing during clinical development. The principles of ICH Q8, ICH Q9, and ICH Q12 can also be applied when developing analytical procedures. This guideline also complements the ICH Q2, i.e., Validation of Analytical procedures.
The development goal is to obtain an analytical procedure fit for its intended purpose, i.e., to measure an attribute or attributes of the analyzed material with the needed specificity/selectivity, accuracy, and precision over the reportable range. Per the ICH Q14 guideline, the development of analytical procedures involves two main approaches: 1. the minimal approach and 2. the enhanced approach.1 These approaches provide a framework for developing methods for analyzing pharmaceuticals and other regulated products.
Minimal Approach
- Robustness Testing: The emphasis is on establishing a simple, robust, well-defined analytical procedure. This procedure should be suitable for its intended purpose without excessive optimization.
- Method Suitability: The procedure should demonstrate suitability for its intended application by evaluating parameters such as specificity, linearity, accuracy, precision, and detection limits.
- Reference Standards: Proper reference standards should ensure accuracy and reliability.
- Stress Testing: Stress testing may be conducted to assess the stability-indicating method’s ability to detect degradation products.
- Minimum Testing: mainly focusing on conducting the minimum testing necessary to ensure the method’s reliability and suitability.
Enhanced Approach
- Design of Experiments (DoE): Statistical tools and DoE principles are applied to optimize and understand the method’s critical parameters systematically.
- Method Development and Optimization: The method is optimized more extensively, considering factors like sample matrix, robustness, and ruggedness.
- Quality by Design (QbD): The enhanced approach aligns with the principles of QbD, which involves developing a comprehensive understanding of the method’s critical attributes to ensure its performance over time.
- Risk Assessment: A thorough risk assessment identifies and prioritizes method variables affecting performance.
- Life Cycle Management: The enhanced approach considers the entire life cycle of the analytical method, including continuous monitoring and improvement.
It’s important to note that the specific content and requirements for analytical procedure development may vary depending on the regulatory agency (e.g., FDA, EMA) and the type of product being analyzed.
Important elements of the ICH Q14 guidelines
Analytical Procedure Lifecycle
The ICH Q14 emphasizes a lifecycle approach with three stages: Procedure Design, Procedure Performance Qualification (PPQ), and Continued Procedure Performance Verification (CPV).
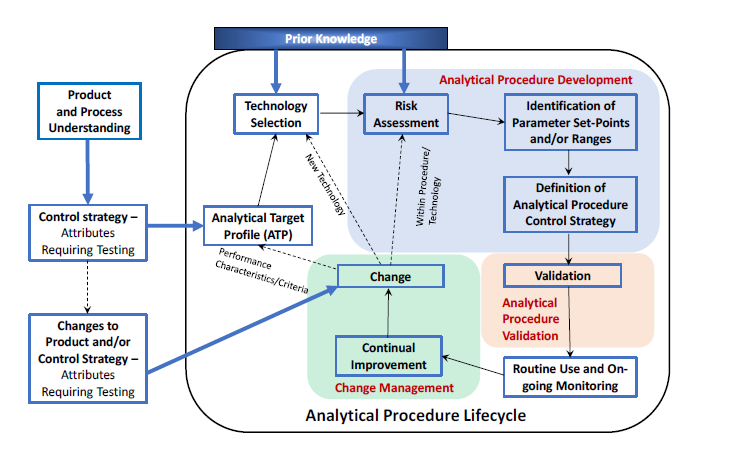
Analytical Target Profile (ATP)
An ATP is a foundation for analytical procedure development by specifying the intended purpose, attributes to be measured, and performance characteristics. It plays a crucial role in guiding technology selection and validation.
Knowledge and Risk management
Knowledge management involves internal (company’s experience) and external (scientific publications, established principles) sources. The ICH Q14 encourages using quality risk management to minimize the risk of analytical procedure performance issues.
Evaluation of Robustness and Parameter ranges
Robustness evaluation assesses a procedure’s ability to perform as expected under normal conditions. Robustness is tested by deliberate variations of analytical procedure parameters. Prior knowledge and risk assessment can inform the selection of parameters to investigate during the robustness study1. Parameter ranges can be established through univariate or multivariate experiments. Risk assessment and prior knowledge should be used to identify parameters, attributes, and appropriate associated ranges that will be investigated experimentally. Categorical variables (e.g., different instruments) can also be considered part of the experimental design1. Parameter ranges are usually subject to regulatory approval.
Analytical Procedure Control Strategy
A control strategy ensures the procedure is performed consistently during routine use. The strategy includes analytical procedure parameters to control and system suitability tests (SST). Sample suitability assessments may also be necessary. In line with ICH Q12, applicants may define established conditions (ECs) for an analytical procedure. ECs are proposed and justified by the applicant and approved by the regulatory authorities.ECs can focus on performance criteria, analytical principles, and parameter ranges.
Lifecycle Management and Post-Approval Changes
Changes to analytical procedures can occur during the product’s lifecycle, and these changes must be managed effectively. Risk assessment is crucial in determining the extent of changes and the associated reporting category. Bridging strategies may be necessary to demonstrate the procedure’s continued suitability after changes.
Details of all the above-mentioned elements can be found in the ICH Q14 guideline.

How can companies use and benefit from the ICH Q14 guideline?
Companies in the pharmaceutical industry can use and benefit from the ICH Q14 guideline in several ways:
- Enhanced Method Development: By following the guidelines principles, companies can develop analytical methods better suited to their specific products and processes, leading to more robust and efficient methods.
- Improved Method Validation: Q14 provides guidance on acceptance criteria for method performance, emphasizing a science-based approach. This ensures that methods are validated appropriately, reducing the risk of method failures and inaccurate results.
- Risk-Based Approach: The guideline encourages risk assessment in method development and validation. Companies can identify and mitigate potential risks early in the process, leading to higher-quality methods and reduced regulatory issues.
- Quality by Design (QbD): Q14 encourages the application of QbD principles to analytical procedures. This proactive approach helps ensure that methods are designed with quality in mind from the outset, reducing the need for post-approval changes.
- Lifecycle Management: Implementing a lifecycle management program for analytical procedures, as recommended in Q14, allows companies to monitor and optimize methods throughout a product’s lifecycle. This can lead to cost savings and improved product quality.
- Flexibility in Post-Approval Changes: The guideline provides a framework for making changes to analytical procedures post-approval based on a risk-based approach. Companies can implement changes more efficiently while maintaining product quality and compliance.
- Global Regulatory Compliance: Following ICH guidelines, including Q14, helps companies align with international regulatory expectations. This can simplify regulatory approval and facilitate global market access for pharmaceutical products.
- Resource Efficiency: By developing better-suited methods for their products, companies can potentially reduce resource-intensive activities like retesting and method optimization.
- Data Integrity: Following Q14 ensures that data generated through analytical procedures are accurate, reliable, and compliant with regulatory requirements, reducing the risk of data integrity issues.
- Continuous Improvement: The guideline promotes a culture of continuous improvement in analytical procedures. Companies can regularly review and optimize methods, increasing efficiency and product quality.
- Facilitates Technology Transfer: Q14 guides method transfer between laboratories, facilitating smoother technology transfer processes within a company or to contract organizations.
- Scientific Advancements: As analytical science advances, Q14 provides a framework for adopting new technologies and methodologies, keeping companies at the forefront of analytical capabilities.
The ICH Q14 guideline offers a comprehensive approach to analytical procedure development, validation, and lifecycle management. Companies that embrace its principles can benefit from improved method quality, reduced risks, better regulatory compliance, and overall operational efficiency in the pharmaceutical industry.
How can companies implement the ICH Q14 guideline?
- Implementing the ICH Q14 guideline for analytical procedure development and revision involves a systematic approach. Here’s a step-by-step guide on how companies can implement this guideline effectively:
- Assessment of Current Practices: Evaluate your current analytical procedures, methods, and validation processes to identify gaps and areas where Q14 principles can be applied.
- Set Up an Analytical Target Profile (ATP): For each analytical procedure, define an ATP that outlines the method’s objectives, critical parameters, and acceptance criteria. This serves as a foundation for method development.
- Adopt Quality by Design (QbD) Principles: Apply QbD concepts to method development, including risk assessment, design of experiments (DoE), and the identification of critical method attributes (CMAs) and critical process parameters (CPPs).
- Method Development: Develop new analytical procedures or revise existing ones using the ATP and QbD principles. Focus on optimizing the method for reliability and robustness.
- Method Validation: Conduct method validation based on the principles outlined in ICH Q2(R1). Ensure that the validation protocol includes scientifically justified acceptance criteria.
- Continued Procedure Performance Verification (CPV): Implement a CPV plan to monitor the ongoing performance of analytical procedures. Review data regularly to ensure the method remains suitable for its intended purpose.
- Change Management: Develop a change control strategy that aligns with the risk-based approach outlined in Q14. Clearly define when changes to analytical procedures require revalidation.
- Lifecycle Management: Establish a lifecycle management program for analytical methods, including periodic method reviews and updates. Ensure that methods remain current and optimized.
- Analytical Method Transfer: If transferring methods between laboratories or sites, follow the guidance in Q14, including conducting a risk assessment to determine the extent of revalidation required.
- Documentation and Record-Keeping: Thoroughly document all method development, validation, and lifecycle management activities. Ensure that records are readily accessible for regulatory inspections.
- Training and Communication: Train personnel on the new processes and ensure effective communication regarding implementing Q14 principles within the organization.
- Regulatory Compliance: Work closely with regulatory affairs experts to ensure that your analytical procedures and documentation comply with regional and international regulatory requirements.
- Continuous Improvement: Foster a culture of continuous improvement in analytical methods. Encourage feedback from staff involved in method development and validation to identify areas for enhancement.
- External Expertise: Consider seeking advice or consultation from analytical sciences and regulatory affairs experts to ensure a smooth and compliant implementation of the Q14 guideline.
- Audit and Verification: Regularly audit and verify the implementation of Q14 principles to ensure ongoing compliance and effectiveness.
By following these steps and integrating the principles outlined in the ICH Q14 guideline into your analytical procedures, companies can systematically improve the quality, reliability, and lifecycle management of their analytical methods in the pharmaceutical industry.






