
As the life science industries continue to evolve, the emphasis on regulation, safety, and efficiency is more pronounced than ever. Verifying a company has and follows standard operational procedures (SOPs) addressing each category using the tried-and-true methods of Commissioning, Qualification, and Validation (CQV) ensures compliance with FDA regulations. Yet, navigating the path to comprehending CQV can be overwhelming for some.
Let PSC Biotech help guide you with our practical guide on how to start with CQV.
Understanding CQV
CQV is the process used by pharmaceutical and life science industries to ensure that a facility, its systems, and equipment fit their intended purpose. This process involves specific verification activities being tested against requirements and specifications. ISPE defines the Commissioning process as a systematic approach to the start-up and turnover of facilities, systems, and equipment to end-users and ensuring that user requirements and design specifications are met. The qualification process refers to verifying that a piece of equipment, system, or software meets the requirements and specifications for its intended purpose. While the validation process focuses on providing the specified performance and results in the user requirements.
At a high level, there are eight steps in implementing a CQV at a facility.
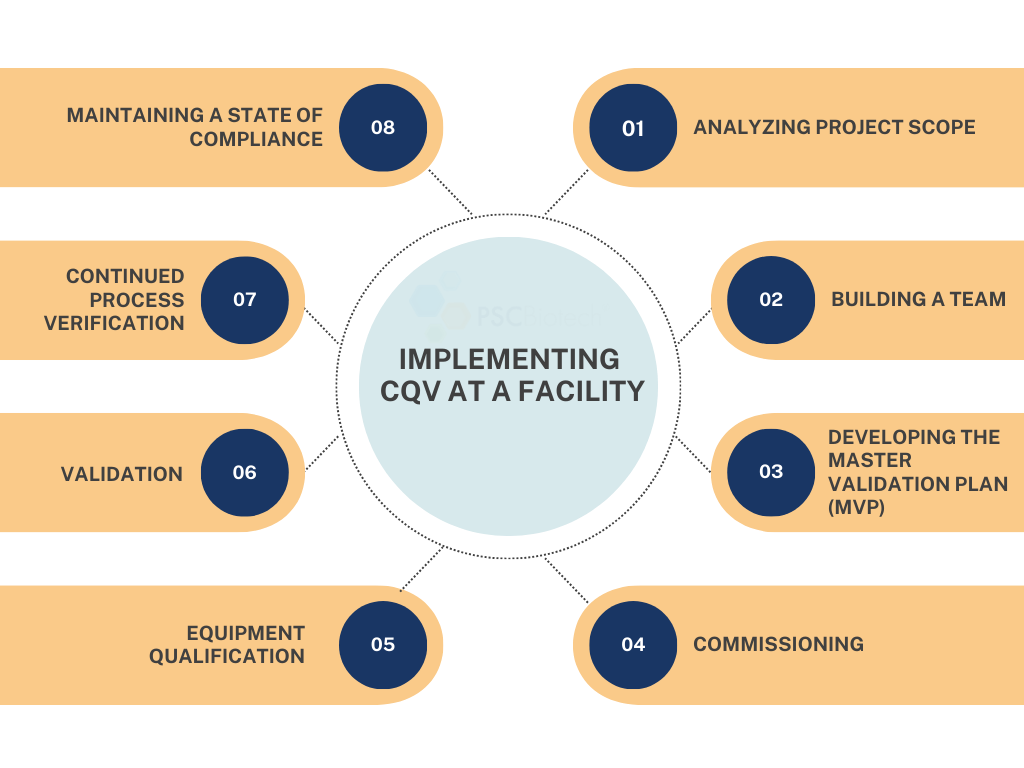
The nine key stages of implementing CQV at a facility.
Step One: Analyzing Project Scope
The first step to implementing a CQV process is to define the project’s scope with a comprehensive understanding of the validation lifecycle and risk mitigation approach. This includes detailing the systems and processes, defining goals, requirements, and specifications identifying potential hazards, and performing assessments (Failure Mode and Effect Analysis (FMEA), cGxP, Data Integrity, 21 CFR Part 11, etc.) that the equipment will be tested against.
Step Two: Building a Team
CQV is a team effort that requires effective coordination and collaboration. Ideally, the team comprises subject matter experts (SMEs) in the type of facility under construction, equipment, and software lifecycle while following FDA Regulations. The team is well balanced by incorporating at least one validation engineer, a project manager, or liaison with the general construction contractor and including the quality assurance lead to oversee the CQV process. Each team member brings a unique perspective and strength to the project’s success.
Step Three: Developing the Master Validation Plan (MVP)
The roadmap for your CQV implementation comes in the form of a Master Validation Plan—a pivotal document giving an overview of the company’s validation strategy, outlining the key roles and responsibilities, timelines, risk assessment procedures, the testing and acceptance criteria, and stating the company programs ensuring a continuing state of validation. Each company may have an SOP defining the content of what in the Master Validation Plan has a template available.
Step Four: Commissioning
Commissioning will be conducted to ensure the facility is correctly designed and built. This will be confirmation of the utilities (air, water, HVAC, power, etc.) performed per design. This testing is not considered Good Manufacturing Practices (GMP), as it does not focus on product impact.
Step Five: Equipment Qualification
The next step of equipment qualification is essential in ensuring finished goods are delivered under repeatable parameters. It includes the Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). DQ provides document verification that the design of the new equipment will result in a system suitable for the intended use. IQ confirms that the equipment is installed correctly and verifies the design specifications. OQ ensures the machine operates per functional specifications. Lastly, PQ confirms that the equipment consistently performs to user specifications in production situations. Some company SOPs require all these documents, while others allow flexibility by only performing a limited number of qualifications. Including the Quality Assurance (QA) department in those discussions is the best.
Step Six: Validation
Process Validation can be defined as generating documented evidence that processes can consistently produce finished goods meeting quality specifications. This will round out the qualification activities and release the equipment for use.
Step Seven: Continued Process Verification
Once the facility has been validated per the MVP, the next focus is to ensure that the process remains controlled during routine production. This is called Continued Process Verification (CPV). It consists of continued assessments of the parameters and attributes within the control strategy as defined in the process design and refined at the end of process qualification.
Step Eight: Maintaining a State of Compliance
Finally, congruent with maintaining FDA Regulations, it’s critical to regularly review and audit your validation protocols and processes to confirm compliance, especially considering any changes made to the systems or process. The quality unit of the company typically performs this.
We will cover other systems and processes such as validation for software, data integrity, computer systems, and so forth, which will be discussed in our upcoming blogs.
Here are some current CQV industry guideline references for further reading:
- FDA 21 CFR Part 210
- FDA 21 CFR Part 211,
- ISPE Baseline Guide 5 Commissioning and Qualification (Second Edition)
- ISPE GAMP 5, A Risk-Based Approach to Compliant GxP Computerized Systems
- The U.S. FDA Process Validation: General Principles and Practices
- ICH Q6A and Q6B Specifications
- ICH Q7, Good Manufacturing Practice
- ICH Q9 Quality Risk Management
- ICH Q10, Pharmaceutical Quality System
- ICH Q11, Development and Manufacture of Drug Substances
- ICH Q12, Lifecycle Management
- ASTM E 2500 Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment
Our Commissioning, Qualification, and Validation Engineers and Project Managers are available to lead and assist you at any level in your project needs.






